Eco-Friendly Photocatalytic Transformation of Greenhouse Gas CO2 into Precious CH4 Fuel via Cu-Deposited Black TiO2 under Simulated Sunlight Irradiation
Ⓒ The Korean Environmental Sciences Society. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Hereunder, the eco-friendly photocatalytic CO2 transformation capability of Cu-deposited black TiO2 (Cu/BTiO2) was evaluated to investigate if this photocatalyst proceeds the thermodynamically- and kinetically-satisfactory CO2 transformation into CH4. The clustered Cu-deposited BTiO2 (Cu/BTiO2) and Cu/BTiO2 architectures revealed noticeable photocatalytic CO2 transformation abilities, whereas the pristine TiO2 and BTiO2 catalysts displayed no significant photocatalytic CO2 transformation abilities. Especially, the photocatalytic CO2 transformation rates of a representative Cu/BTiO2 architecture were 104, 209, 272, 322, and 361 μmol/g at the irradiation times of 2, 4, 6, 8, and 10 h, respectively, while the photocatalytic CO2 transformation rates of Cu/BTiO2 were 61, 139, 217, 270, and 309 μmol/g at the same irradiation times, respectively. The promoted photocatalytic CO2 transformation ability of the Cu/BTiO2 architecture was assigned to the excellent electron‒hole separation tendency, which was verified by the photoluminescence analysis. The composition ratio of Cu incorporated into BTiO2 in the Cu/BTiO2 architectures was crucial in CH4 generation. In addition, the Cu/BTiO2 architecture displayed eminent photodurability, which was verified by the consecutive experiment cycle, and the mechanistic process for CO2 transformation into CH4 via the Cu/BTiO2 architecture was established. The electronic framework of the Cu/BTiO2 architecture was established on the basis of its band gap and valence band value. Conclusively, the Cu/BTiO2 architecture is an outstanding tool for thermodynamically- and kinetically-satisfactory photocatalytic CO2 transformation into CH4 that application under simulated sunlight irradiation.
Keywords:
Clustered Cu, Thermodynamically-satisfactory, Kinetically-satisfactory, Experiment cycle, Photodurability1. Introduction
With the ever-increasing combustion of hydrocarbon-containing fuels, the greenhouse gas concentration in the atmosphere is gradually elevating, which leads to substantial problems about the detrimental impacts on the climate and energy shortage crisis (Lewis, 2019; Liao et al., 2019; Dong et al., 2020; Li et al., 2021; Zhang et al., 2021; Tang et al., 2023; Majumdar et al., 2024). This crucial environmental issue prompts the urgent development of the effective techniques for the mitigation of CO2 emission into the atmosphere. Typically, much efforts have been given to explore the thermocatalytic techniques for CO2 transformation into precious chemicals (Bermejo-López et al., 2019; Chen et al., 2020; Xu et al., 2020). However, in the conventional thermocatalytic CO2 transformation method, hydrogen gas is employed as reducing material to produce hydrogen atoms for the formation of reactive electrons, which causes explosive risks and high costs (Bermejo-López et al., 2019; Chen et al., 2020; Xu et al., 2020). Fortunately, artificially-mimicked photosynthesis using the solar light-activated photocatalytic process has been recently suggested as an optimistic approach to address the energy shortage as well as atmospheric CO2 level mitigation by transforming CO2 into precious fuels such as CH4 and other hydrocarbons (Chen et al., 2019; Choi et al., 2019; Li et al., 2019; Pan et al., 2019; Xie et al., 2019; Zhang et al., 2021; Cheng et al., 2024). In addition, CO2 is potentially be converted into valuable chemicals that are utilized in various industrial processes (Pan et al., 2019; Xie et al., 2019; Zhang et al., 2021). Semiconductor–based photocatalysts have been most widely utilized for CO2 transformation to precious materials due to their prominent photodurabilty and appropriate electron structures for CO2 reduction reaction (Jiang et al., 2018; Wu et al., 2019; Guo et al., 2020; Xiong et al., 2020; Zeng et al., 2021; Zhao et al., 2021). In spite of tremendous efforts, the photocatalytic CO2 transformation technique is yet challenging due to its thermodynamically and kinetically unfavorable reaction pathways (Li et al., 2021; Zhang et al., 2021). Thus, high-performance photocatalytic techniques with thermodynamically and kinetically favorable CO2 transformation pathway are necessary to overcome the aforementioned problems.
To accomplish the thermodynamically-approving CO2 transformation pathway into a precious fuel CH4, the conduction band of a photocatalyst must be negative than the CO2/CH4 reduction potential of −0.24 eV (Fang and Wang, 2018; Zhou et al., 2018; Wang et al., 2021). Among the popularly employed photocatalysts, TiO2 has received tremendous interests in its photocatalytic application to CO2 transformation because of its prominent photostability and higher conduction band value (−0.61 eV) than the CO2/CH4 reduction potential (Wang et al., 2019a; Wang et al., 2019b; Xiong et al., 2020; Zhang et al., 2021). Nonetheless, pristine TiO2 shows an unacceptable photocatalytic capability and low quantum yields under visible-light irradiation due to its fast carrier recombination rate and wide bandgap, respectively, significantly inhibiting its actual utilization to the photocatalytic CO2 transformation under visible-light irradiation (Shi et al., 2019; Zhang et al., 2021). This limitation may be overcome by modifying TiO2 into black TiO2, which can increase optical efficiencies of TiO2 due to the disordered structure, transformed electron properties, and a range of defect groups (Ullattil et al., 2018; Rajaraman et al., 2020).
To achieve kinetically-satisfactory CO2 transformation pathway into CH4, the carrier separation efficiency of the photocatalyst should be considered (Fang and Wang, 2018; Zhou et al., 2018; Wang et al., 2021). The surface alteration of photocatalysts by depositing a pertinent metal cocatalyst can promote its separation efficiency of carriers due to the high carrier transportability at interfaces of photocatalyst‒cocatalyst (Shen et al., 2019; Yi et al., 2019; Ishii et al., 2020). The non-precious metal copper (Cu) can be utilized as a potential cocatalyst because of its prominent charge acceptance nature, which promotes the carrier separation rates (Chen et al., 2018; Maldonado et al., 2018; Liu et al., 2019; Ge et al., 2020). Markedly, compared to conventional aggregated metal cocatalysts, metal cocatalysts show greater probability for providing more reactive spots and cost moderation, prompting their utilization in the advancement of potential metal-deposited photocatalysts (Wei et al., 2019; Gao et al., 2020; Li et al., 2020).
In this study, the photocatalytic CO2 transformation capability of Cu-deposited black TiO2 (Cu/BTiO2) was evaluated to examine whether this photocatalyst follows the thermodynamically-and kinetically-satisfactory CO2 transformation into CH4. For comparison, selected reference samples (i.e., pristine TiO2, black TiO2 (BTiO2), and conventional aggregated Cu-deposited BTiO2 (Cu/BTiO2)) were evaluated for the photocatalytic CO2 transformation into CH4. Further, probable mechanistic insight into the photocatalytic CO2 transformation into CH4 via Cu/BTiO2 was investigated, and the photostability of this photocatalyst was assessed through a recycling test.
2. Methods
2.1. Photocatalyst fabrication
The BTiO2 sample was fabricated by reducing pristine TiO2 using a temperature-programmed electric furnace under H2 (30 mL/min)/Ar (470 mL/min) gas flow conditions. In brief, pristine TiO2 powder (4.0 g) was dispersed in an alumina boat and placed at the central site of a quartz tube in the electric furnace. The reduction process was carried out in the furnace heated to 400oC and kept for 5 h. The reducing compounds were cleaned with deionized water, filtered, and dried at 85oC for 10 h to obtain the BTiO2 photocatalyst.
The Cu/BTiO2 samples was fabricated by photodepositing Cu onto the BTiO2 powder. For this procedure, BTiO2 powder (0.1 g) was suspended into deionized water (0.1 L) to prepare solution A. In addition, Cu(NO3)2·3H2O (0.95 g) was dispersed to deionized water (0.25 L) to prepare solution B. Subsequently, solution B (1, 1.2, 1.4, 1.6, 1,8, or 2 mL) was slowly added to solution A, after which this solution was stirred for 0.5 h to obtain a homogeneous solution. The mixture was then transferred to a Pyrex reactor equipped with a quartz window. After closing all openings of the reactor, the solution was purged using pure CO2 for 0.5 h and irradiated by a 350-W Xe for 4 h. Finally, the suspension was vacuum-treated and washed using ethanol, and the resultant precipitate was dried and ground to obtain Cu/BTiO2 sample. The Cu/BTiO2 samples prepared using Cu(NO3)2·3H2O solution amounts of 1, 1.2, 1.4, 1.6, 1.8, and 2 mL were denoted as Cu/BTiO2-1, Cu/BTiO2-1.2, Cu/BTiO2-1.4, Cu/BTiO2-1.6, Cu/BTiO2-1.8, and Cu/BTiO2-2, respectively. Furthermore, the Cu/BTiO2 sample was fabricated by following the above procedure; in this case, the mixed solution in the reactor was purged using Ar instead of CO2 gas.
2.2. Photocatalyst characterization
The fabricated materials were surveyed by X-ray diffraction (XRD) spectroscopy, transmission electron microscopy(TEM), high-resolution transmission electron microscopy (HRTEM), UV–visible spectrophotometry, X-ray photoelectron spectroscopy (XPS), and photoluminescence (PL) emission spectroscopy. XRD patterns were recorded for 2θ = 10°–80° using a Rigaku D/max-2500 diffractometer (Tokyo, Japan). The morphology of the materials was inspected using a Hitachi 7700 instrument operated at 150 kV (TEM, Hitachi, Japan). The HRTEM patterns were captured using a FEI Titan G2 ChemiSTEM Cs Probe instrument (Hillsboro, OR, USA). The UV–visible spectra were inspected using the CARY 5G Varian spectrophotometer (Cary, NC, USA). The XPS spectra were afforded using the PHI Quantera SXM instrument (Chanhassen, MN, USA. The high-resolved PL emission spectra were afforded using an Action Research Spectra Pro 2150i spectrophotometer (Princeton, NJ, USA).
2.3. Measurement of the photoelectric currents
Transient photoelectric current measurements were performed on the Ivium Technologies electrochemical workstation with triple-electrode cells. Indium tin oxide (ITO) covered by a photocatalyst was regarded as the work electrode. Ag/AgCl immersed in KCl solution and Pt film were used as the reference and counter electrodes, correspondingly. The Na2SO4 (0.5 M) solution was utilized as the electrolytic material. The current signals of the photocatalysts were determined via a 350-W Xe light source. The as-fabricated photocatalyst (0.02 g) was dispersed in Nafion solution (5 wt%). The solution was continuously stirred to afford a slurry-type sample, after which this slurry was placed on the ITO plate with a covered area of 1.5 cm2. Finally, the sample-covered ITO plate was dried at 85°C for 10 h and the photocurrent generated on this ITO plate was measured using the Ivium Technologies electrochemical workstation.
2.4. Photocatalytic CO2 transformation into CH4
The photocatalytic capability of the as-constructed materials for CO2 transformation into CH4 was determined utilizing a secured stainless steel chamber (100 mL) fitted with a quartz window for light penetration. Summarily, a sample photocatalyst was dispersed in a solution of Na2SO4 (0.7 g), NaHCO3 (0.5 g), and deionized water (0.06 L), after which the dispersion was ultrasonicated for 0.5 h. The solution was then bubbled with super-pure CO2 for 0.5 h to saturate it with CO2 as well as to exclude oxygen from the solution. Subsequently, all openings of the stainless steel chamber were closed, and the chamber was illuminated through the 350-W Xe light source placed at the sunlight supplier. Gas samples (500 μl) were regularly taken through the sampling port of the stainless steel chamber utilizing a gastight micro-syringe. The collected samples were qualitatively and quantitatively determined employing a gas chromatography (GC-Plus, Shimazu, Japan) installed with a thermal conductivity detection system. The quantitative investigation of the collected compounds was carried out using the calibration curve, which was established by analyses of standard gases. Furthermore, three control experiments were carried out: 1) CO2 transformation into CH4 in the presence of light but without the addition of a photocatalyst; 2) CO2 transformation into CH4 in the presence of a photocatalyst but without light; and 3) CO2 transformation into CH4 in the presence of light and a photocatalyst under CO2-free conditions. The control experiments were carried out utilizing the abovementioned CO2 transformation process.
3. Results and discussion
3.1. Material characteristics
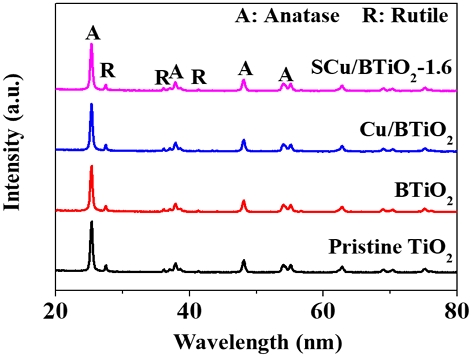
The crystalline characteristics of pristine TiO2, BTiO2, Cu/BTiO2, and Cu/BTiO2-1 are presented in their XRD spectra (Fig. 1). All TiO2-containing materials presented revealed exclusive signs indexed to the anatase and rutile TiO2 crystals at ~25.3o (101) and ~27.3o (110), respectively (Naldoni et al., 2019; Rajaraman et al., 2020). Noticeably, the XRD patterns of the TiO2-containing materials showed similar intensities at their matched 2θ number, implying that their crystal properties are not substantially altered during their fabrication. Nonetheless, the Cu-related signs did not appear in the XRD spectra of the Cu/BTiO2-1.6, probably owing to the unmeasurable weight of Cu impregnated onto the BTiO2.
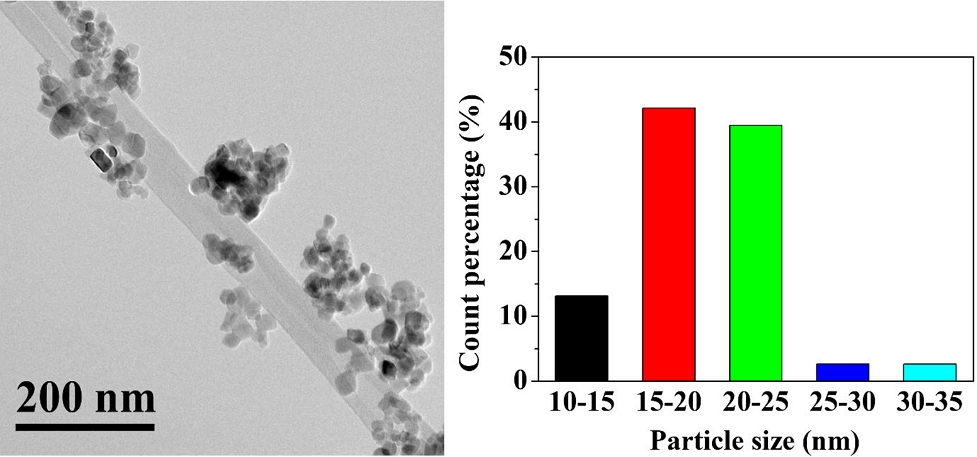
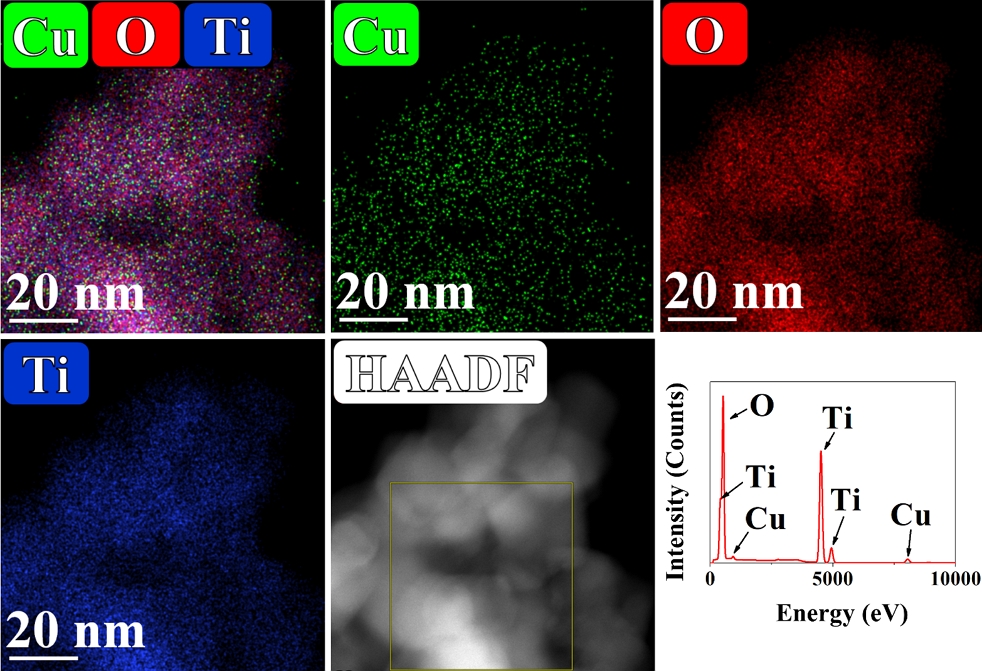
The hetero-structural and surface properties of as-constructed Cu/BTiO2-1.6 inspected via TEM and HRTEM are presented in Figs. 2 and 3. Especially, Fig. 2 represents the TEM result and size distribution of Cu/BTiO2-1.6. The TEM image of the material illustrates nanoparticles with diameter range of 10‒35, which is comparable to that of P25 TiO2 diameter (10‒30 nm), which are consistent with the results of previous studies Wang et al., 2018 ; Naldoni et al., 2019; She et al., 2020). Besides, the HRTEM mapping figure presents three images of Ti, O, and Cu in Cu/BTiO2-1.6 and their corresponding EDS peaks (Fig. 3), demonstrating the successful incorporation of Cu and BTiO2.
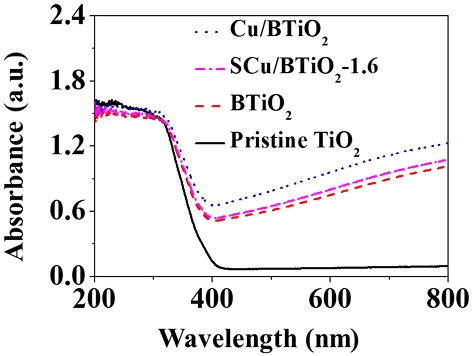
The optical characteristics of pristine TiO2, BTiO2, Cu/BTiO2, and Cu/BTiO2-1.6 inspected via UV‒vis absorption spectroscopy are shown in Fig. 4. The three modified materials (BTiO2, Cu/BTiO2, and Cu/BTiO2-1.6) presented a red-shift in light harvest relative to pristine TiO2, which results from the elevated visible-light absorption of the former materials. Besides, the visible-light absorption rates of Cu or Cu-incorporated materials (Cu/BTiO2 and Cu/BTiO2-1.6) were greater than that of BTiO2, ascribing to the eminent charge acceptance nature of Cu and Cu, which promotes the carrier separation rates (Liu et al., 2019; Gao et al., 2020; Ge et al., 2020; Li et al., 2020). On the basis of the converted Tauc plots of the UV-vis absorption spectra, the bad edge of pristine TiO2, BTiO2, Cu/BTiO2, and Cu/BTiO2-1.6 were 3.19, 3.05, 2.87, and 2.91 eV, respectively.
3.2. Photocatalytic CO2 transformation into CH4
The photocatalytic CO2 transformation rates over as-fabricated catalysts were investigated under sunlight exposure. Prior to conducting the main photocatalytic CO2 transformation experiments, three control experiments were carried out to demonstrate the scientific hypothesis that precious gas CH4 is solely generated by the photocatalytic transformation of CO2. Table 1 exhibits the control experiment results for photocatalytic CO2 transformation into CH4. First, CH4 was unobserved in the presence of light but without the addition of a photocatalyst. Second, CH4 was unobserved in the presence of a photocatalyst but without light. Lastly, CH4 was yet unobserved in the presence of light and a photocatalyst under CO2-free conditions. As such, the control experiments demonstrate the abovementioned scientific hypothesis.
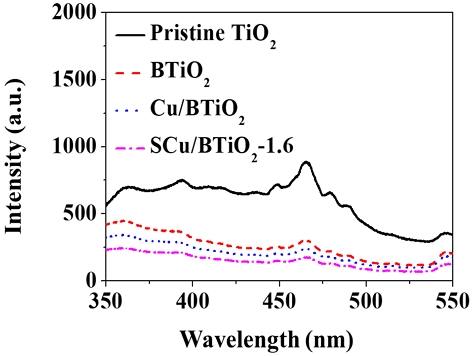
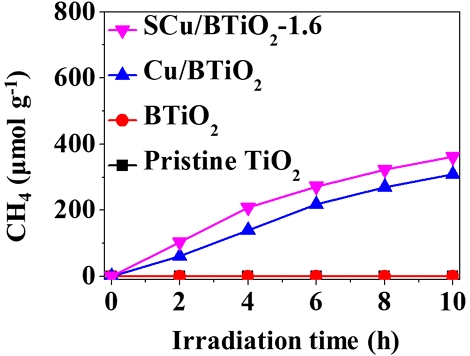
The photocatalytic CO2 transformation rates determined using pristine TiO2, BTiO2, Cu/BTiO2, and Cu/BTiO2-1.6 are shown in Fig. 5. The pristine TiO2 and BTiO2 catalysts revealed no significant photocatalytic CO2 transformation abilities. Contrarily, Cu/BTiO2 and Cu/BTiO2-1.6 displayed noticeable photocatalytic CO2 transformation abilities: The photocatalytic CO2 transformation rates of Cu/BTiO2 were 61, 139, 217, 270, and 309 μmol/g at the irradiation times of 2, 4, 6, 8, and 10 h, respectively; the photocatalytic CO2 transformation rates of Cu/BTiO2-1.6 were 104, 209, 272, 322, and 361 μmol/g at the irradiation times of 2, 4, 6, 8, and 10 h, respectively. The greatest photocatalytic CO2 transformation ability of Cu/BTiO2-1.6 is assigned to the excellent electron‒hole separation tendency. This statement is demonstrated by Fig. 6, which shows the smaller PL emission signal for Cu/BTiO2-1.6 relative to those of three reference catalysts (pristine TiO2, BTiO2, and Cu/BTiO2) since a small PL signal indicates a great electron‒hole separation tendency (Xie et al., 2020; Xiong et al., 2020).
Time-series CH4 transformation rates obtained from pristine TiO2, BTiO2, Cu/BTiO2, and Cu/BTiO2-1.6.
To ensure that the composition ratio of Cu incorporated into BTiO2 in the Cu/BTiO2 architectures is crucial in CH4 generation, the CO2 transformation activities of Cu/BTiO2 architectures with different Cu loadings. As shown in Fig. 7, the CO2 transformation activities of Cu/BTiO2 architectures are ordered as follows: Cu/BTiO2-1.6 > Cu/BTiO2-1.8 > Cu/BTiO2-2.0 > Cu/BTiO2-1.4 > Cu/BTiO2-1.2 > Cu/BTiO2-1.0. This pattern of photocatalysts in CO2 transformation activities are ascribed to their electron‒hole separation tendencies, which is demonstrated by the PL emission signals of the surveyed catalysts (Fig. 8). As such, these results imply the existence of an optimum Cu composition incorporated into BTiO2 in the Cu/BTiO2 architectures.
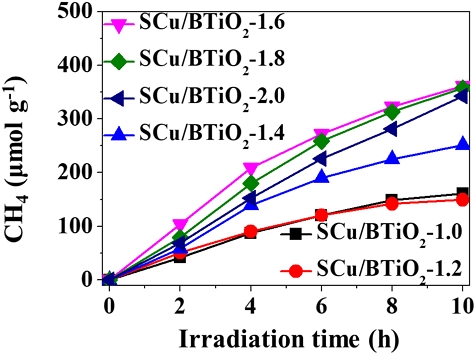
Time-series CH4 transformation rates obtained from Cu/BTiO2-1, Cu/BTiO2-1.2, Cu/BTiO2-1.4, Cu/BTiO2-1.6, Cu/BTiO2-1.8, and Cu/BTiO2-2.
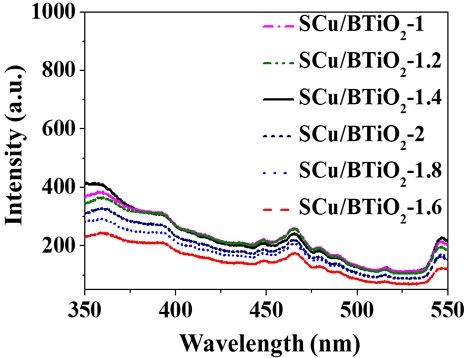
Photoluminescence emission spectra obtained from Cu/BTiO2-1, Cu/BTiO2-1.2, Cu/BTiO2-1.4, Cu/BTiO2-1.6, Cu/BTiO2-1.8, and Cu/BTiO2-2.
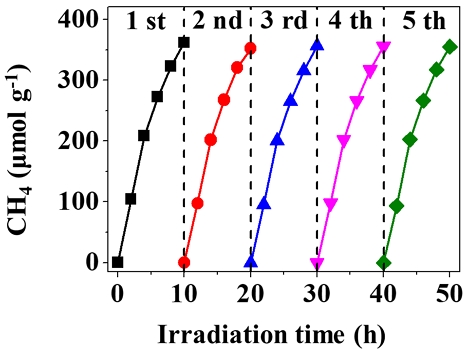
To verify the durability and stability of the Cu/BTiO2 architectures, the time program of CH4 generation employing the Cu/BTiO2-1.6 architecture was attained under the same experiment conditions. As shown in Fig. 9, the CH4 generation ratio is constant under prolonged light irradiation up to 50 h. Since this CO2 transformation activity was evaluated in a closed reaction device, a just small variation in CO2 transformation activity was observable. To survey the photostability of the architecture, five experiment cycles were carried out. regarding each experiment cycle, the sample powder was washed and positioned in the experimental system. Fig. 9 represents that the content of CH4 increases with the irradiation time, and no recognizable reduction in CH4 generation after the final cycle is observable. Consequently, the as-prepared architecture kept eminent photostability during the prolonged experiment.
4. Conclusions
In view of this paper, the photocatalytic CO2 transformation capability of Cu/BTiO2 was evaluated to investigate if this photocatalyst proceeds the thermodynamically-and kinetically-satisfactory CO2 transformation into CH4. The Cu/BTiO2 and Cu/BTiO2 architectures revealed noticeable photocatalytic CO2 transformation abilities, whereas the pristine TiO2 and BTiO2 catalysts displayed no significant photocatalytic CO2 transformation abilities. Moreover, the Cu/BTiO2 architecture exhibited greater photocatalytic CO2 transformation ability compared with that of the Cu/BTiO2 architecture, which was ascribed to the promoted electron‒hole separation tendency of the former. Another important finding is that the composition ratio of Cu incorporated into BTiO2 in the Cu/BTiO2 architectures is crucial in CH4 generation. The Cu/BTiO2 architecture also revealed eminent photodurability, which was demonstrated by the consecutive experiment cycle. Consequently, the promoted photocatalytic CO2 transformation capability of Cu/BTiO2 verifies its utilization as an efficient tool in photocatalytic CO2 transformation.
REFERENCES
-
Bermejo-López, A., Pereda-Ayo, B., González-Marcos, J. A., González-Velasco, J. R., 2019, Mechanism of the CO2 storage and in situ hydrogenation to CH4. Temperature and adsorbent loading effects over Ru-CaO/Al2O3 and Ru-Na2CO3/Al2O3 catalysts, Appl. Catal. B, 256, 117845.
[https://doi.org/10.1016/j.apcatb.2019.117845]

-
Chen, W., Wang, Y., Liu, S., Gao, L., Mao, L., Fan, Z., Shangguan, W., Jiang, Z., 2018, Non-noble metal Cu as a cocatalyst on TiO2 nanorod for highly efficient photocatalytic hydrogen production, Appl. Surf. Sci., 445, 527–534.
[https://doi.org/10.1016/j.apsusc.2018.03.209]

-
Chen, W., Han, B., Tian, C., Liu, X., Liang, S., Deng, H., Lin, Z., 2019, MOFs-derived ultrathin holey Co3O4 nanosheets for enhanced visible light CO2 reduction, Appl. Catal. B, 244, 996–1003.
[https://doi.org/10.1016/j.apcatb.2018.12.045]

-
Chen, X., Li, Q., Li, J., Chen, J., Jia, H., 2020, Modulating charge separation via in situ hydrothermal assembly of low content Bi2S3 into UiO-66 for efficient photothermocatalytic CO2 reduction, Appl. Catal. B, 270, 118915.
[https://doi.org/10.1016/j.apcatb.2020.118915]

-
Cheng, M., Gao, B., Zheng, X., Wu, W., Kong, W., Yan, P., Wang, Z., An, B., Zhang, Y., Li, Q., Xu, Q., 2024, CO2-assisted rapid synthesis of porphyrin-based Bi-MOFs for photocatalytic CO2 reduction: An Efficient strategy for carbon cycle, Appl. Catal. B, 353, 124097.
[https://doi.org/10.1016/j.apcatb.2024.124097]

-
Choi, M., Ahn, C. Y., Lee, H., Kim, J. K., Oh, S. H., Hwang, W., Yang, S., Kim, J., Kim, O. H., Choi, I., 2019, Bi-modified Pt supported on carbon black as electro-oxidation catalyst for 300 W formic acid fuel cell stack, Appl. Catal. B, 253, 187–195.
[https://doi.org/10.1016/j.apcatb.2019.04.059]

-
Dong, H., Meng, X. B., Zhang, X., Tang, H. L., Liu, J. W., Wang, J. H., Wei, J. Z., Zhang, F. M., Bai, L. L., Sun, X. J., 2020, Boosting visible-light hydrogen evolution of covalent-organic frameworks by introducing Ni-based noble metal-free co-catalyst, Chem. Eng. J., 379, 122342.
[https://doi.org/10.1016/j.cej.2019.122342]

-
Fang, Y., Wang, X., 2018, Photocatalytic CO2 conversion by polymeric carbon nitrides, Chem. Commun., 54, 5674–5687.
[https://doi.org/10.1039/C8CC02046A]

-
Gao, C., Low, J., Long, R., Kong, T., Zhu, J., Xiong, Y., 2020, Heterogeneous single-atom photocatalysts: Fundamentals and applications, Chem. Rev., 120, 12175–12216.
[https://doi.org/10.1021/acs.chemrev.9b00840]

-
Ge, H., Zhang, B., Liang, H., Zhang, M., Fang, K., Chen, Y., Qin, Y., 2020, Photocatalytic conversion of CO2 into light olefins over TiO2 nanotube confined Cu clusters with high ratio of Cu+, Appl. Catal. B, 263, 118133.
[https://doi.org/10.1016/j.apcatb.2019.118133]

-
Guo, Q., Fu, L., Yan, T., Tian, W., Ma, D., Li, J., Jiang, Y., Wang, X., 2020, Improved photocatalytic activity of porous ZnO nanosheets by thermal deposition graphene-like g-C3N4 for CO2 reduction with H2O vapor, Appl. Surf. Sci., 509, 144773.
[https://doi.org/10.1016/j.apsusc.2019.144773]

-
Ishii, T., Anzai, A., Yamamoto, A., Yoshida, H., 2020, Calcium zirconate photocatalyst and silver cocatalyst for reduction of carbon dioxide with water, Appl. Catal. B, 277, 119192.
[https://doi.org/10.1016/j.apcatb.2020.119192]

-
Jiang, Z., Zhang, X., Yuan, Z., Chen, J., Huang, B., Dionysiou, D. D., Yang, G., 2018, Enhanced photocatalytic CO2 reduction via the synergistic effect between Ag and activated carbon in TiO2/AC-Ag ternary architecture, Chem. Eng. J., 348, 592–598.
[https://doi.org/10.1016/j.cej.2018.04.180]

-
Lewis, N. S., 2019, A Prospective on energy and environmental science, Energy Enviro. Sci., 12, 16‒18.
[https://doi.org/10.1039/C8EE90070A]

-
Li, X., Yu, J., Jaroniec, M., Chen, X., 2019, Cocatalysts for selective photoreduction of CO2 into solar fuels, Chem. Rev., 119, 3962–4179.
[https://doi.org/10.1021/acs.chemrev.8b00400]

-
Li, L., Chang, X., Lin, X., Zhao, Z. J., Gong, J., 2020, Theoretical insights into single-atom catalysts, Chem. Soc. Rev., 49, 8156–8178.
[https://doi.org/10.1039/D0CS00795A]

-
Li, J., Huang, B., Guo, Q., Guo, S., Peng, Z., Liu, J., Tian, Q., Yang, Y., Xu, Q., Liu, Z., Liu, B., 2021, Van der Waals heterojunction for selective visible-light-driven photocatalytic CO2 reduction, Appl. Catal. B, 284, 119733.
[https://doi.org/10.1016/j.apcatb.2020.119733]

-
Liao, G. F., Gong, Y., Zhang, L., Gao, H. Y., Yang, G. J., Fang, B. Z., 2019, Semiconductor polymeric graphitic carbon nitride photocatalysts: The ‘‘holy grail’’ for the photocatalytic hydrogen evolution reaction under visible light, Energy Environ. Sci., 12, 2080–2147.
[https://doi.org/10.1039/C9EE00717B]

-
Liu, C., Nauert, S. L., Alsina, M. A., Wang, D., Grant, A., He, K., Weitz, E., Nolan, M., Graya, K. A., Notestein, J. M., 2019, Role of surface reconstruction on Cu/TiO2 nanotubes for CO2 conversion, Appl. Catal. B, 255, 117754.
[https://doi.org/10.1016/j.apcatb.2019.117754]

-
Maldonado, M. I., López-Martín, A., Colón, G., Peral, J., Martínez-Costa, J. I., Malato, S., 2018, Solar pilot plant scale hydrogen generation by irradiation of Cu/TiO2 architectures in presence of sacrificial electron donors, Appl, Catal. B, 229, 15–23.
[https://doi.org/10.1016/j.apcatb.2018.02.005]

-
Majumdar, S. S., Moses-DeBusk, M., Deka, D. J., Kidder, M. K., Thomas, C. R., Pihl, J. A., 2024, Impact of Mg on Pd-based methane oxidation catalysts for lean-burn natural gas emissions control, Appl. Catal. B, 341, 123253.
[https://doi.org/10.1016/j.apcatb.2023.123253]

-
Naldoni, A., Altomare, M., Zoppellaro, G., Liu, N., Kment, Š., Zbořil, R., Schmuki, P., 2019, Photocatalysis with reduced TiO2: From black TiO2 to cocatalyst-free hydrogen production, ACS Catal., 9, 345–364.
[https://doi.org/10.1021/acscatal.8b04068]

-
Pan, F., Li, B., Deng, W., Du, Z., Gang, Y., Wang, G., Li, Y., 2019, Promoting electrocatalytic CO2 reduction on nitrogen-doped carbon with sulfur addition, Appl. Catal. B, 252, 240–249.
[https://doi.org/10.1016/j.apcatb.2019.04.025]

-
Rajaraman, T. S., Parikh, S. P., Gandhi, V. G., 2020, Black TiO2: A Review of its properties and conflicting trends, Chem. Eng. J., 389, 123918.
[https://doi.org/10.1016/j.cej.2019.123918]

-
She, P., Rao, H., Guan, B., Qin, J. S., Yu, J., 2020, Spatially separated bifunctional cocatalysts decorated on hollow-structured TiO2 for enhanced photocatalytic hydrogen generation, ACS Appl. Mater. Interfaces, 12, 23356–23362.
[https://doi.org/10.1021/acsami.0c04905]

-
Shen, R., Jiang, C., Xiang, Q., Xie, J., Li, X., 2019, Surface and interface engineering of hierarchical photocatalysts, Appl. Surf. Sci., 471, 43–87.
[https://doi.org/10.1016/j.apsusc.2018.11.205]

-
Shi, Q., Li, Z., Chen, L., Zhang, X., Han, W., Xie, M., Yang, J., Jing, L., 2019, Synthesis of SPR Au/BiVO4 quantum dot/rutile-TiO2 nanorod array architectures as efficient visible-light photocatalysts to convert CO2 and mechanism insight, Appl. Catal. B, 244, 641–649.
[https://doi.org/10.1016/j.apcatb.2018.11.089]

-
Tang, Y., L, Y., Bao, W., Yan, W., Zhang, J., Huang, Y., Li, H., Wang, Z., Liu, M., Yu, F., 2023, Enhanced dry reforming of CO2 and CH4 on photothermal catalyst Ru/ SrTiO3, Appl. Catal. B, 338, 123054.
[https://doi.org/10.1016/j.apcatb.2023.123054]

-
Ullattil, S. G., Narendranath, S. B., Pillai, S. C., Periyat, P., 2018, Black TiO2 nanomaterials: A Review of recent advances, Chem. Eng. J., 343, 708–736,
[https://doi.org/10.1016/j.cej.2018.01.069]

-
Wang, L., Duan, S., Jin, P., She, H., Huang, J., Lei, Z., Zhang, T., Wang, Q., 2018, Anchored Cu(II) tetra(4-carboxylphenyl)porphyrin to P25 (TiO2) for efficient photocatalytic ability in CO2 reduction, Appl. Catal. B, 239, 599‒608.
[https://doi.org/10.1016/j.apcatb.2018.08.007]

-
Wang, S. L., Xu, M., Peng, T. Y., Zhang, C. X., Li, T., Hussain, I., Wang, J. Y., Tan, B. E., 2019a, Porous hypercrosslinked polymer-TiO2-graphene architecture photocatalysts for visible-light-driven CO2 conversion, Nat. Commun., 10, 676–686.
[https://doi.org/10.1038/s41467-019-08651-x]

-
Wang, R., Shen, J., Sun, K., Tang, H., Liu, Q., 2019b, Enhancement in photocatalytic activity of CO2 reduction to CH4 by 0D/2D Au/TiO2 plasmon heterojunction, Appl. Surf. Sci., 493, 1142–1149.
[https://doi.org/10.1016/j.apsusc.2019.07.121]

-
Wang, Y., Huang, H., Zhang, Z. Wang, C., Yang, Y., Li, Q., Xu, D., 2021, Lead-free perovskite Cs2AgBiBr6@g-C3N4 Z-scheme system for improving CH4 production in photocatalytic CO2 reduction, Appl. Catal. B, 282, 119570.
[https://doi.org/10.1016/j.apcatb.2020.119570]

-
Wei, T., Zhu, Y., Wu, Y., An, X., Liu, L. M., 2019, Effect of single-atom cocatalysts on the activity of faceted TiO2 photocatalysts, Langmuir, 35, 391–397.
[https://doi.org/10.1021/acs.langmuir.8b03488]

-
Wu, J., Feng, Y., Li, D., Han, X., Liu, J., 2019, Efficient photocatalytic CO2 reduction by PeO linked g-C3N4/TiO2-nanotubes Z-scheme architectures, Energy, 178, 168–175.
[https://doi.org/10.1016/j.energy.2019.04.168]

-
Xie, C., Niu, Z., Kim, D., Li, M., Yang, P., 2020, Surface and interface control in nanoparticle catalysis, Chem. Rev., 120, 1184–1249.
[https://doi.org/10.1021/acs.chemrev.9b00220]

-
Xiong, Z., Xu, Z., Li, Y., Dong, L., Wang, J., Zhao, J., Chen, X., Zhao, Y., Zhao, H., Zhang, J., 2020, Incorporating highly dispersed and stable Cu+ into TiO2 lattice for enhanced photocatalytic CO2 reduction with water, Appl. Surf. Sci., 507, 145095.
[https://doi.org/10.1016/j.apsusc.2019.145095]

-
Xu, S., Chansai, S., Shao, Y., Xu, S., Wang, Y. C., Haigh, S., Mu, Y., Jiao, Y., Stere, C. E., Chen, H., Fan, X., Hardacre, C., 2020, Mechanistic study of non-thermal plasma assisted CO2 hydrogenation over Ru supported on MgAl layered double hydroxide, Appl. Catal. B, 268, 118752.
[https://doi.org/10.1016/j.apcatb.2020.118752]

-
Yi, J., Li, H., Gong, Y., She, X., Song, Y., Xu, Y., Deng, J., Yuan, S., Xu, H., Li, H., 2019, Phase and interlayer effect of transition metal dichalcogenide cocatalyst toward photocatalytic hydrogen evolution: The case of MoSe2, Appl. Catal. B, 243, 330–336.
[https://doi.org/10.1016/j.apcatb.2018.10.054]

-
Zeng, C., Zeng, Q., Dai, C., Zhang, L., Hu, Y., 2021, Synergistic effect of surface coated and bulk doped carbon on enhancing photocatalytic CO2 reduction for MgIn2S4 microflowers, Appl. Surf. Sci., 542, 148686.
[https://doi.org/10.1016/j.apsusc.2020.148686]

-
Zhang, H., Li, Y., Wang, J., Wu, N., Sheng, H., Chen, C., Zhao, J., 2021, An Unprecedent hydride transfer pathway for selective photocatalytic reduction of CO2 to formic acid on TiO2, Appl. Catal. B, 284, 11692.
[https://doi.org/10.1016/j.apcatb.2020.119692]

-
Zhao, X., Guan, J., Li, J., Li, X., Wang, H., Huo, P., Yan, Y., 2021, CeO2/3D g-C3N4 heterojunction deposited with Pt cocatalyst for enhanced photocatalytic CO2 reduction, Appl. Surf. Sci., 537, 147891.
[https://doi.org/10.1016/j.apsusc.2020.147891]

-
Zhou, M., Wang, S., Yang, P., Huang, C., Wang, X., 2018, Boron carbon nitride semiconductors decorated with CdS nanoparticles for photocatalytic reduction of CO2, ACS Catal. 8, 4928–4936.
[https://doi.org/10.1021/acscatal.8b00104]

School of Architecture, Civil, Environmental and Energy Engineering, Kyungpook National Universityeastcamp@knu.ac.kr
JIGU Environment and Consulting Inc.enviland@naver.com
JIGU Environment and Consulting Inc.ssho37@naver.com
School of Architecture, Civil, Environmental and Energy Engineering, Kyungpook National University wkjo@knu.ac.kr