
Evaluation of Skin Irritation and Moisture Retention of a Cream Formulation Containing Batryticatus Bombyx Extract
Ⓒ The Korean Environmental Sciences Society. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Environmental factors have a significant impact on skin health, leading to increased research on protecting the skin barrier from external stressors. Batryticatus Bombyx refers to silkworms that have been infected by the fungus Beauveria bassiana and subsequently died. It has long been used as an ingredient in traditional Korean medicine. Recently, scientific research has actively explored its anti-inflammatory, antioxidant, anti-allergic, and antimicrobial properties. However, there is limited scientific evidence regarding the safety and efficacy of these extracts for topical applications. In this study, we aim to evaluate the skin-irritation potential and the 24 h moisturizing efficacy of a cream formulation containing Batryticatus Bombyx extract. The primary objectives were to confirm the non-irritating nature of the formulation and assess its ability to enhance skin moisture levels over a prolonged period. A skin irritation test conducted on 34 female participants showed no adverse reactions, confirming that the formulation was non-irritating. In the moisture retention test, skin hydration significantly improved immediately after application and after 24 h compared to that in untreated areas. Additionally, the cream demonstrated superior performance in maintaining skin moisture for 24 h compared with the control product. The results indicate that cream formulations containing Batryticatus Bombyx extract are safe for topical use and effective in enhancing skin hydration. These findings suggest that the extract could be a valuable natural resource for developing moisturizing skincare products that specifically target dry and sensitive skin exposed to environmental stressors.
Keywords:
Batryticatus Bombyx, Skin barrier, Functional cosmetic, Environmental factor1. Introduction
The environment and skin barrier protection are closely linked, with growing concern over the effects of environmental pollution and climate change on skin health (Bocheva et al., 2023). Factors like fine dust and air pollution can accumulate on the skin, causing inflammation and damaging the skin barrier, which leads to increased sensitivity and moisture loss (He et al., 2024). Additionally, prolonged ultraviolet (UV) exposure weakens the barrier function and accelerates oxidative stress, contributing to premature aging and pigmentation (Faria and Andrade, 2024). Climate changes, such as cold, dry weather and extreme temperature shifts, further impair the skin's ability to retain moisture, weakening its protective function (Seurat et al., 2021; Bai et al., 2024). As a result, there is increasing interest in developing functional cosmetics using natural resources to protect the skin from these environmental stressors.
Batryticatus Bombyx is a traditional medicinal ingredient extracted from silkworms that have died after being infected by the fungus Beauveria bassiana. Historically used in Korean herbal medicine, it has recently garnered scientific attention for its potential skin benefits. Studies have reported that Batryticatus Bombyx extract possesses anti-inflammatory, antioxidant, anti-allergic, and antimicrobial properties (Hu et al., 2017; Wang et al., 2024). It reduces inflammation by inhibiting cytokine production, protects against oxidative damage from UV exposure due to its rich flavonoid and polyphenol content, and modulates immune responses to alleviate allergic reactions (Hu et al., 2017). While there is substantial scientific evidence supporting these benefits, research on its safety and efficacy for topical skin application remains limited. This study evaluated whether a cream formulation containing Batryticatus Bombyx extract induces irritation when applied to human skin and assessed its efficacy in improving moisture retention. The findings explored its potential as a functional cosmetic ingredient for protecting the skin barrier.
2. Materials and Methods
2.1. Cream formulation utilizing Batryticatus Bombyx extract
The cream formulation utilizing Batryticatus Bombyx extract was manufactured by DHU Medicos Co., Ltd (Gyeongsan, Gyeongsangbuk-do, Republic of Korea). The full list of ingredients in the cream formulation is as follows: Batryticatus Bombyx extract, water, squalane, caprylic/capric triglyceride, glycerin, dipropylene glycol, cyclopentasiloxane, butylene glycol, niacinamide, 1,2-hexanediol, cetyl alcohol, glyceryl stearate, polyglyceryl-3 methylglucose distearate, glyceryl stearate citrate, betaine, PEG-100 stearate, panthenol, sodium carbomer, ammonium acryloyldimethyltaurate/VP copolymer, allantoin, ethylhexylglycerin, caprylyl glycol, adenosine, carbomer, prunus amygdalus amara (Bitter almond) kernel oil, camellia sinensis leaf extract, sodium hyaluronate, disodium EDTA, Vanilla Planifolia fruit extract, Pyrus Malus (apple) leaf extract, Jasminum Officinale (Jasmine) flower/leaf extract, myristyl alcohol, stearyl alcohol, pentylene glycol, hydrolyzed collagen, sodium hyaluronate crosspolymer, hydrolyzed hyaluronic acid, hyaluronic acid, hydrolyzed sodium hyaluronate (Table 1).
2.2. Human skin primary irritation test
This evaluation was conducted by the P&K Skin Clinical Research Center (Seoul, Republic of Korea). The study was conducted in accordance with the Regulations on the Evaluation of Functional Cosmetics, Ministry of Food and Drug Safety Notification No. 2021-55, and the standard operating procedures (SOP) of the P&K Skin Clinical Research Center. A human skin primary irritation test was conducted on 34 participants aged 20-55 who were healthy and free from skin conditions (Table 2). The cream was applied to a flat, undamaged area on the back, excluding the spine. Visual assessments were conducted using Frosch & Kligman guidelines and the Draize method to evaluate skin reactions (Frosch and Kligman, 1979; Draize et al., 1994). After patch application, skin responses were observed one hour and 24 hours post-removal. The primary evaluation focused on determining the skin irritation index to confirm the safety of the cream. Participants provided informed consent and were monitored for any adverse reactions during the study period.
2.3. Human application test for surface moisture improvement and moisture retention
This evaluation was conducted by the P&K Skin Clinical Research Center (Seoul, Republic of Korea). The study was conducted in accordance with the Regulations on the Evaluation of Functional Cosmetics, Ministry of Food and Drug Safety Notification No. 2021-55, and the standard operating procedures (SOP) of the P&K Skin Clinical Research Center. A human moisture improvement and moisture retention test was conducted on 11 participants aged 20-55 who were healthy and free from skin conditions (Table 1). The evaluation included several assessment categories: instrumental evaluation using a corneometer CM825 to measure 24-hour moisture retention, and a global assessment of efficacy survey where participants provided feedback. For safety evaluation, researchers combined visual assessments of the application site with participants' survey responses. Additional observational assessments included demographic data (gender, date of birth, age), health status checks to confirm suitability for the study, and medical history regarding symptoms, onset dates, and treatment history. A survey was also conducted to evaluate participants' product preference. The study involved two visits: Visit 1 included informed consent, participant screening, baseline and immediate post-application skin measurements, and an adverse reaction survey. Visit 2 involved follow-up measurements and assessments 24 hours after application, along with another adverse reaction survey.
2.4. Statistical analysis
To determine the significance of the measurements compared to baseline values, the statistical analysis was performed using SPSS version 27.0. A significance level of p < 0.05 was considered statistically significant within a 95% confidence interval, and p-values were rounded to three decimal places. Continuous variables were summarized using means and standard deviations, while categorical variables were summarized using frequencies and percentages. For datasets with more than three repeated measurements, normality was assessed. Parametric analysis was conducted using repeated measures ANOVA, followed by post-hoc analysis with the Bonferroni method. For non-parametric analysis, the Friedman test was applied, followed by pairwise comparisons using the Wilcoxon signed-rank test with Bonferroni adjustment for multiple comparisons. Between-group comparisons were conducted using raw data and analyzed with repeated measures ANOVA to assess differences among groups.
3. Results
3.1. Evaluation of human skin primary irritation test for cream formulation containing Batryticatus Bombyx extract
The cream formulation containing Batryticatus Bombyx extract was patch-tested for 24 hours, and skin reactions were visually assessed by the investigator at 1 hour and 24 hours after patch removal to determine the skin irritation index and degree of irritation. No adverse reactions or use of concomitant medications were reported during the study period. As a result, the test product containing Batryticatus Bombyx extract was classified as non-irritating (Table 3).
3.2. Efficacy evaluation of surface moisture improvement for the cream formulation containing Batryticatus Bombyx extract
A survey was conducted to assess the effects of a single application of the cream formulation containing Batryticatus Bombyx extract on surface moisture improvement and 24-hour moisture retention. The results indicated that the cream demonstrated surface moisture improvement and 24-hour moisture retention effects rated as average or above. These findings suggest that the cream formulation containing Batryticatus Bombyx extract may serve as an effective material for supporting the skin barrier by enhancing hydration and moisture retention (Table 4).
3.3. Efficacy evaluation of moisture retention for the cream formulation containing Batryticatus Bombyx extract
In the moisture retention test, the cream formulation containing Batryticatus Bombyx extract-treated area showed statistically significant improvement in hydration levels immediately after application and maintained increased hydration at 24 hours post-application compared to the untreated site. Additionally, the cream demonstrated superior moisture retention compared to a control product (Fig. 1). Therefore, the cream formulation containing Batryticatus Bombyx extract is confirmed to be an effective material for protecting the skin from environmental stressors.
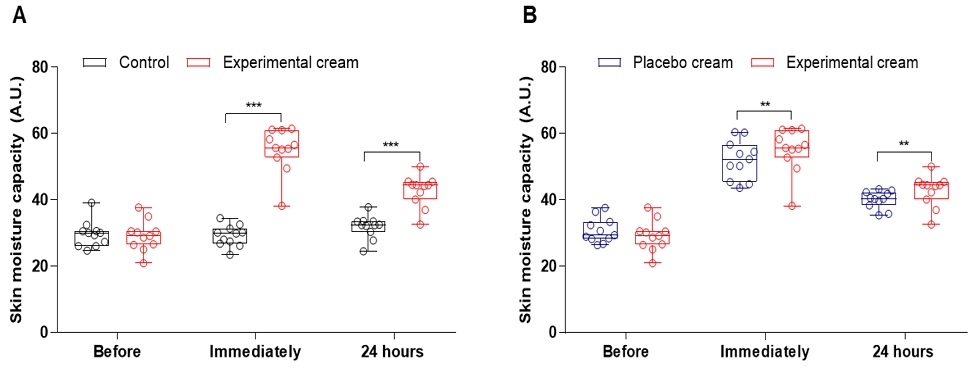
Moisture retention measurement for the cream formulation containing Batryticatus Bombyx extract. (A) Measurement results of 24 h moisture retention compared to untreated skin and (B) measurement results of 24 h moisture retention compared to the control product. The control product was formulated with the same ingredients as the cream formulation, excluding Batryticatus Bombyx extract. ***p < 0.001 by control vs experimental cream; **p < 0.01 by placebo cream vs experimental cream. Control, untreated cream; Placebo cream, cream formulation excluding Batryticatus Bombyx extract; Experimental cream, cream formulation containing Batryticatus Bombyx extract.
4. Discussion
Environmental factors play a significant role in compromising skin health, as they can disrupt the skin's natural barrier and accelerate damage (Singh et al., 2024). Pollutants like fine dust not only settle on the skin but also penetrate deeper layers, triggering inflammatory responses and oxidative stress (Sundas et al., 2024). Similarly, UV radiation exacerbates these effects by promoting free radical formation, which breaks down essential skin components like collagen and lipids (Bai et al., 2024). Fluctuating environmental conditions, such as extreme dryness or humidity, further challenge the skin’s ability to maintain hydration, leading to a compromised barrier and increased vulnerability to irritants. These concerns have fueled a growing demand for innovative skincare solutions that leverage natural resources to reinforce the skin barrier and counteract the cumulative impact of environmental aggressors (Olivero-Verbel et al., 2024). The results of this study highlight the potential benefits of Batryticatus Bombyx extract in skincare formulations. The absence of irritation among all participants supports the hypothesis that Batryticatus Bombyx extract is safe for topical application, even on sensitive skin types.
The improvement in skin hydration observed in this study can be attributed to the properties of Batryticatus Bombyx extract. This extract contains a complex mixture of bioactive compounds, including antioxidants and natural moisturizing factors, which work synergistically to enhance the skin’s moisture retention capabilities. By strengthening the lipid barrier and minimizing transepidermal water loss, the extract supports prolonged hydration. Additionally, it is believed that humectant-like components in the extract attract and bind water to the skin's surface, promoting sustained hydration. These mechanisms collectively explain the observed enhancement in skin moisture retention following the application of the formulation containing Batryticatus Bombyx extract. Furthermore, the study's comparison with a control product indicates that the test cream provided superior hydration benefits. This supports the potential of Batryticatus Bombyx extract as an effective ingredient in moisturizing products. Future studies could explore the long-term effects of this extract, particularly in products designed for dry or sensitive skin.
5. Conclusions
This study demonstrates that a cream formulation containing Batryticatus Bombyx extract is both safe and effective for enhancing skin hydration. These findings contribute to the growing body of evidence supporting the use of natural ingredients in cosmetics and may encourage the development of more products utilizing traditional medicinal extracts. However, this study has several limitations that should be acknowledged. First, the small sample size used for the hydration efficacy tests limits the statistical power and generalizability of the findings. As this research was designed as a preliminary investigation, the sample size was intentionally kept small to gather exploratory insights while minimizing participant burden. Second, the short evaluation duration focused on short-term hydration effects, which may not fully capture the long-term benefits or performance of the cream formulation. To address these limitations, future studies will incorporate a larger participant pool and extend the evaluation period to assess both immediate and long-term effects. These follow-up investigations will provide more robust and comprehensive data to validate the preliminary findings and strengthen the overall conclusions. By identifying and addressing these limitations, we aim to enhance the reliability and applicability of our research in subsequent studies. Furthermore, we will analyze the active components of Batryticatus Bombyx extract responsible for enhancing moisture retention and continue to study the mechanisms by which it improves skin conditions through skin barrier protection.
Acknowledgments
This work was supported by the Development of Sustainable Application for Standard Herbal Resources (KSN1822320) and Technology upgrade of Batryticatus Bombyx for improvement of skin barrier function (PSN2111640) from the Korea Institute of Oriental Medicine, Republic of Korea.
REFERENCES
-
Bai, L., Li, J., Guo, B., Cai, R., Zhao, C., Guo, Y., Wang, Y., Jiang, G., 2024, Percutaneous penetration and dermal exposure risk assessment of UV absorbents in sunscreens and isolation cosmetics, Environ. Health (Wash), 2, 541-552.
[https://doi.org/10.1021/envhealth.4c00039]

-
Bocheva, G., Slominski, R. M., Slominski, A. T., 2023, Environmental air pollutants affecting skin functions with systemic implications, Int. J. Mol. Sci., 24, 10502.
[https://doi.org/10.3390/ijms241310502]

- Draize, J., Woodard, G., Calvery, H., 1994, Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes, J. Pharm. Exp. Ther., 82, 377-390.
-
Faria, A. V. S., Andrade, S. S., 2024, Decoding the impact of ageing and environmental stressors on skin cell communication, Biogerontology, 26, 3.
[https://doi.org/10.1007/s10522-024-10145-3]

-
Frosch, P. J., Kligman, A. M., 1979, The soap chamber test: A New method for assessing the irritancy of soaps, J. Am. Acad. Dermatol., 1, 35-41.
[https://doi.org/10.1016/S0190-9622(79)70001-6]

-
He, T., Tang, W., Chen, J., Xie, J., Weng, Z., Deng, D., Zhang, C., Wang, X., 2024, Hydrogel-based treatment of house dust mite-induced atopic dermatitis through triple cleaning of mites, bacteria, and ROS-related inflammation, ACS Appl. Mater. Interfaces, 16, 33121-33134.
[https://doi.org/10.1021/acsami.4c05435]

-
Hu, M., Yu, Z., Wang, J., Fan, W., Liu, Y., Li, J., et al., 2017, Traditional uses; Origins; Chemistry and pharmacology of Bombyx Batryticatus: A Review, Molecules, 22, 1779.
[https://doi.org/10.3390/molecules22101779]

-
Olivero-Verbel, J., Quintero-Rincón, P., Caballero-Gallardo, K., 2024, Aromatic plants as cosmeceuticals: Benefits and applications for skin health, Planta, 260, 132.
[https://doi.org/10.1007/s00425-024-04550-8]

-
Seurat, E., Verdin, A., Cazier, F., Courcot, D., Fitoussi, R., Vié, K., Desauziers, V., Momas, I., Seta, N., Achard, S., 2021, Influence of the environmental relative humidity on the inflammatory response of skin model after exposure to various environmental pollutants, Environ. Res., 196, 110350.
[https://doi.org/10.1016/j.envres.2020.110350]

-
Singh, N., Wigmann, C., Vijay, P., Phuleria, H. C., Kress, S., Majmudar, G., Kong, R., Krutmann, J., Schikowski, T., 2024, Combined effect of ambient temperature and relative humidity on skin aging phenotypes in the era of climate change: Results from an indian cohort study, Dermatitis.
[https://doi.org/10.1089/derm.2024.0301]

-
Sundas, A., Contreras, I., Mujahid, O., Beneyto, A., Vehi, J., 2024, The effects of environmental factors on general human health: A scoping review, Healthcare (Basel), 12, 2123.
[https://doi.org/10.3390/healthcare12212123]

-
Wang, S. S., Xu, H., Ge, A. Q., Yang, K. L., He, Q., Ge, J. W., 2024, Bombyx Batryticatus extract activates coagulation factor XII to promote angiogenesis in rats with cerebral ischemia/reperfusion injury, J. Ethnopharmacol., 319(Pt 1), 117081.
[https://doi.org/10.1016/j.jep.2023.117081]

Herbal Medicine Resources Research Center, Korea Institute of Oriental Medicine gpark@kiom.re.kr
Herbal Medicine Resources Research Center, Korea Institute of Oriental Medicineqp1015@kiom.re.kr
