Anti-Inflammatory Effects of Juncus leschenaultii Extract on HaCaT Cells: Regulation of TSLP, TARC, and Aquaporin-3 Expression
Ⓒ The Korean Environmental Sciences Society. All rights reserved.
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Juncus leschenaultii, a medicinal plant traditionally used for its anti-inflammatory properties, has recently gained attention owing to its potential therapeutic effects on inflammatory skin disorders. The present study evaluated the effects of J. leschenaultii extract on key inflammatory markers in spontaneously transformed human keratinocyte cell cultures (HaCaTs) cells, a human keratinocyte model. The extract significantly reduced the expression of thymic stromal lymphopoietin (TSLP) and thymus and activation-regulated chemokine (TARC), which are critical mediators of inflammation in keratinocytes. Moreover, the extract enhanced the expression of aquaporin-3 (AQP3), a vital protein involved in the maintenance of skin hydration and barrier function. These findings suggest that J. leschenaultii extract mitigates inflammation by downregulating TSLP and TARC as well as promotes skin health by upregulating AQP3. This dual action highlights its potential as a novel therapeutic agent for managing inflammatory skin conditions such as atopic dermatitis. Further studies are required to elucidate the underlying mechanisms and to explore its clinical applications.
Keywords:
Juncus leschenaultia, HaCaT keratinocyte, TSLP, TARC, Aquaporin-31. Introduction
Inflammatory skin conditions, such as atopic dermatitis (AD), are characterized by immune dysregulation and epithelial barrier dysfunction, leading to chronic inflammation and impaired skin health (Boguniewicz and Leung, 2011). Among the key players in these conditions are thymic stromal lymphopoietin (TSLP) and thymus and activation-regulated chemokine (TARC), which are well-established markers of inflammatory responses in keratinocytes (Hijnen et al., 2013; Kim and Lee, 2021). Targeting these pathways holds significant therapeutic potential for managing inflammatory skin disorders.
Juncus leschenaultii, a traditional medicinal plant, has garnered attention for its potential pharmacological activities, including anti-inflammatory effects (Park et al., 2016; Lee and Lee, 2018). However, the underlying mechanisms, particularly its influence on skin-specific inflammation markers, remain largely unexplored. Recent studies have identified aquaporin-3 (AQP3), a water and glycerol channel expressed in keratinocytes, as a critical factor in maintaining skin hydration, promoting wound healing, and modulating inflammatory responses (Sugiyama et al., 2010; Zeng et al., 2020). Dysregulation of AQP3 has been linked to inflammatory skin conditions, suggesting it as a potential therapeutic target.
In this study, we investigated the effects of Juncus leschenaultii extract (JLE) on inflammatory responses in HaCaT cells, a human keratinocyte cell line widely used in skin research. We focused on its ability to regulate the expression of TSLP, TARC, and AQP3. By elucidating these mechanisms, this research aims to provide a scientific basis for the potential use of JLE as a therapeutic agent for inflammatory skin diseases.
2. Materials and Methods
2.1. Plant material
The JLE used in this study was obtained from the Natural Product Central Bank (Ochang, Korea) and prepared using 95% (v/v) methanol as the extraction solvent. The JLE used in this study was sourced from Jeollabuk-do, South Korea. A voucher specimen (KPM037-083) has been deposited as a freeze-dried powder at the Korea Institute of Oriental Medicine (Daejeon, Korea).
2.2. Cell culture
The HaCaT human keratinocyte cell line was obtained from CLS Cell Lines Service GmbH (Eppelheim, Baden-Württemberg, Germany). The cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco BRL, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco BRL) and 1% penicillin- streptomycin (Gibco BRL). The cells were incubated under standard conditions of 37°C and 5% CO2.
2.3. Measurement of cell viability
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8; Dojindo, Japan) assay. HaCaT cells (3 × 103 cells/well) were seeded in a 96-well plate and incubated for 18 hours. The cells were then treated with JLE at concentrations ranging from 1.25 to 10 μg/mL and incubated for an additional 24 hours. Following treatment, 10 μL of CCK-8 reagent was added to each well and incubated for 4 hours. Absorbance was measured at 450 nm using a microplate reader (SpectraMax i3 Multi-Mode Detection Platform, Molecular Devices, USA). Cell viability (%) = (mean absorbance in JLE-treated cells/mean absorbance in untreated control cells) × 100. Extracts were considered non-toxic when cell viability was greater than 90%.
2.4. Protective effect against oxidative stress-induced damage
The protective effects of JLE against oxidative stress-induced cell damage were assessed using the MTT assay. HaCaT cells (3 × 103 cells/well) were seeded in a 96-well plate and incubated for 18 hours. The cells were then treated with JLE at concentrations ranging from 1.25 to 5 μg/mL and incubated for an additional 1 hours. After 1 hours of incubation, oxidative stress was induced by treating the cells with 0.5 mM hydrogen peroxide (H2O2), a known inducer of cell viability reduction, and the cells were incubated for 4 hours. Following the incubation period, the medium was removed, and the formazan crystals formed in each well were dissolved by adding DMSO. The absorbance was measured at 570 nm using a microplate reader. This method allowed for the quantification of cell viability and the evaluation of the protective effects of JLE against oxidative stress-induced damage.
2.5. RNA extraction and real-time gene expression measurement
HaCaT cells (1 × 106 cells/well) were seeded in a 6-well plate and cultured in medium containing 10% FBS for 18 hours. Afterward, the medium was replaced with serum-free DMEM. The cells were pretreated with JLE at various concentrations for 1 hour, followed by stimulation with TNF-α/IFN-γ (10 ng/mL) in each well for 23 hours. After a total of 24 hours, RNA was extracted from the cells using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, USA) and quantified using a NanoDrop spectrophotometer. Equal concentrations of RNA (100 ng/μL) were reverse-transcribed into cDNA using the iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). The synthesized cDNA was then used to analyze the gene expression of TSLP, TARC, and AQP3 by real-time RT-PCR. Real-time PCR was performed using SYBR Green PCR Master Mix. The reaction conditions included an initial denaturation step at 95°C for 5 minutes, followed by 35 cycles of denaturation at 95°C for 30 seconds and annealing/extension at 58°C for 30 seconds. The housekeeping gene GAPDH was used as an internal control for gene expression analysis.
2.6. Immunofluorescence staining
HaCaT cells were seeded on glass coverslips and treated with JLE at concentrations of 1.25–5 μg/mL for 1 hour, followed by stimulation with TNF-α/IFN-γ at 10 ng/mL for 23 hours. After a total of 24 hours, the cells were fixed with 4% paraformaldehyde for 30 minutes, permeabilized with PBS, and blocked with 1% bovine serum albumin in PBS. Subsequently, the cells were incubated with rabbit monoclonal antibodies against TSLP (Santa Cruz Biotechnology, Dallas, TX, USA) at 4°C overnight. The cells were then washed with PBS and incubated with a fluorescently conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 hour. The immunostained cells were mounted using a medium containing 4′,6-diamidino-2-phenylindole (DAPI) and visualized using an Olympus CKX53 Microscope System (Olympus, Tokyo, Japan).
2.7. Statistical analysis
The data were expressed as the mean ± standard error of the mean (SEM) from three independent experiments. Statistical significance of the experimental data was analyzed using GraphPad PRISM software version 7 (GraphPad Software, La Jolla, CA, USA). ANOVA was performed, followed by Dunnett’s multiple comparison test. P-value of less than 0.05 was considered statistically significant.
3. Results
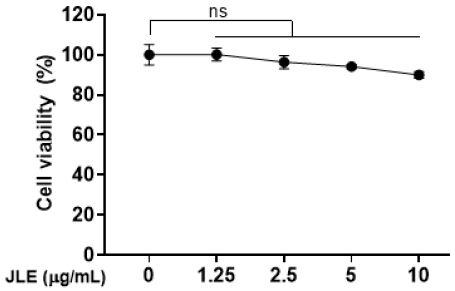
3.1. Effect of JLE on the viability in HaCaT cells
To evaluate the effects of JLE on HaCaT cell viability, the extract was treated at concentrations of 1.25, 2.5, 5, and 10 μg/mL, followed by a CCK-8 assay. The results showed that cell viability remained above 90% at concentrations up to 5 μg/mL, indicating no cytotoxicity. However, cytotoxicity was observed at a concentration of 10 μg/mL (Fig. 1). Based on these findings, subsequent experiments for analyzing anti-inflammatory efficacy were conducted using concentrations of 5 μg/mL or lower.
3.2. Protective effect of JLE against oxidative stress-induced cell damage in HaCaT cells
he protective effects of JLE against oxidative stress-induced cell damage were evaluated in HaCaT cells. The results demonstrated that JLE effectively mitigated H2O2-induced cell damage, with significantly improved cell viability observed at all tested concentrations compared to cells treated with H2O2 alone (Fig. 2). This indicates that JLE exhibits protective effects against oxidative stress by potentially enhancing the cellular defense mechanisms in HaCaT cells.
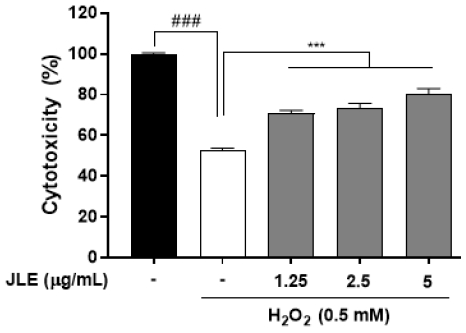
Protective effect of JLE against oxidative stress-induced cell damage in HaCaT cells. Cells were pretreated with JLE at concentrations of 1.25, 2.5, and 5 μg/mL for 1 hour, followed by exposure to 0.5 mM H2O2 for 4 hours to induce oxidative stress. The results are expressed as the mean ± SEM of three independent experiments. ###p<0.001 compared with non-treated cells; ***p<0.001 compared with TNF-α/IFN-γ-treated cells.
3.3. Inhibitory effect of JLE on TSLP expression in HaCaT cells
The effects of JLE on the expression of TSLP were investigated in HaCaT cells. The cells were pretreated with JLE at concentrations of 1.25, 2.5, and 5 μg/mL for 1 hour, followed by stimulation with TNF-α/IFN-γ (10 ng/mL) for 23 hours to induce TSLP expression. TSLP levels were evaluated using real-time RT-PCR and immunofluorescence staining. The results showed that JLE significantly reduced TSLP expression in a concentration-dependent manner compared to the TNF-α/IFN-γ-stimulated control group (Fig. 3A). Immunofluorescence analysis further confirmed the downregulation of TSLP expression, with reduced fluorescence intensity observed in JLE-treated cells (Fig. 3B). These findings indicate that JLE effectively suppresses TSLP expression, suggesting its potential as a therapeutic agent for managing inflammatory skin conditions mediated by TSLP.
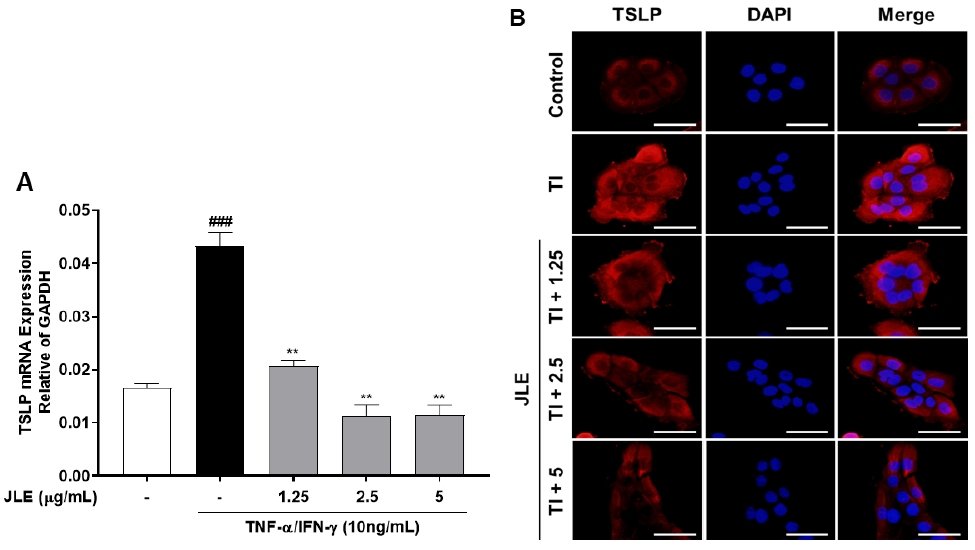
Inhibitory effect of JLE on TSLP expression in HaCaT cells. Cells were pretreated with JLE at concentrations of 1.25, 2.5, and 5 μg/mL for 1 hour, followed by stimulation with TNF-α/IFN-γ (10 ng/mL) for 23 hours to induce TSLP expression. (A) TSLP expression levels were analyzed using real-time RT-PCR. The results are expressed as the mean ± SEM of three independent experiments. ###p <0.001 compared with non-treated cells; **p <0.01 compared with TNF-α/IFN-γ-treated cells. (B) Immunofluorescence microscope was used to detect the TSLP (red) and nucleus (blue). Scale bar: 100 μm.
3.4. Suppression of TARC expression and enhancement of AQP3 expression of JLE in HaCaT cells
The effects of JLE on the expression of TARC and AQP3 were evaluated in HaCaT cells. The results demonstrated that JLE significantly inhibited the TNF-α/IFN-γ-induced upregulation of TARC expression in a concentration-dependent manner (Fig. 4A). In contrast, JLE treatment enhanced the expression of AQP3, which is critical for maintaining skin hydration and barrier function (Fig. 4B). These findings suggest that JLE not only mitigates inflammation by suppressing TARC expression but also promotes skin health by enhancing AQP3 expression, highlighting its dual therapeutic potential in treating inflammatory skin conditions.
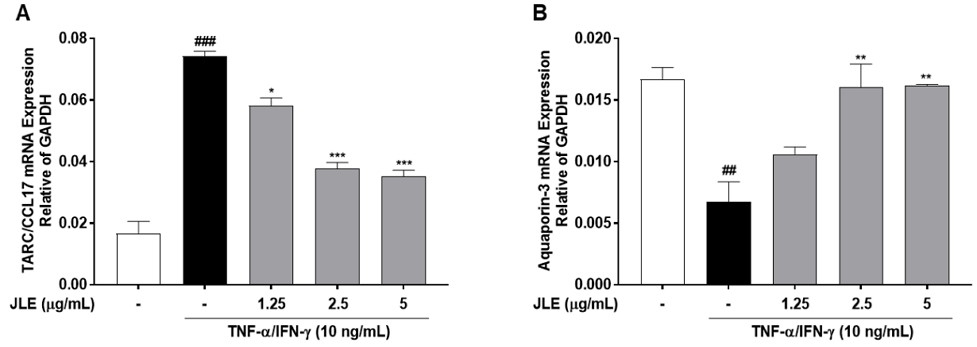
Suppression of TARC expression and enhancement of AQP3 expression of JLE in HaCaT cells. Cells were pretreated with JLE for 1 hour and then stimulated with TNF-α/IFN-γ (10 ng/ml each) for 23 hours. The mRNA expression of (A) TSLP and (B) AQP3 was examined by real-time PCR. Data are represented as mean ± SEM of three independent experiments. #p < 0.01 and ###p < 0.001 compared with non-treated cells; *p < 0.05, **p < 0.01, and ***p < 0.001 compared with TNF-α/IFN-γ-treated cells.
4. Discussion
This study investigated the anti-inflammatory effects of JLE in HaCaT cells, focusing on its regulation of key markers such as TARC, TSLP, and AQP3 (Gallegos-Alcalá et al., 2023; Hu and Zhang, 2022). The findings demonstrate that JLE has significant therapeutic potential for managing inflammatory skin conditions. JLE effectively inhibited the expression of TARC and TSLP, both of which play pivotal roles in the pathogenesis of inflammatory skin disorders, including AD. TSLP acts as an upstream driver of Th2 inflammation by activating dendritic cells and initiating the recruitment of Th2 cells through TARC (Elentner et al., 2009; Uysal et al., 2017). The suppression of these markers by JLE suggests its potential to attenuate the inflammatory cascade, thereby alleviating symptoms of skin inflammation. In addition to its anti-inflammatory properties, JLE enhanced the expression of AQP3, a key protein involved in maintaining skin hydration, wound healing, and barrier function. AQP3 downregulation has been implicated in various skin conditions, including dryness and impaired barrier integrity. By upregulating AQP3, JLE not only addresses inflammation but also supports skin repair and hydration, providing a dual benefit in skin health management.
The dual activity of JLE suppressing inflammatory mediators while enhancing protective factors positions it as a promising candidate for the treatment of complex skin conditions like AD, where inflammation and barrier dysfunction coexist. The concentration-dependent effects observed in this study further underscore its therapeutic potential, as lower concentrations effectively modulated key markers without inducing cytotoxicity. The mechanisms underlying the observed effects likely involve the bioactive compounds in JLE, which warrant further investigation to identify the specific molecules responsible for these activities. Additionally, in vivo studies are needed to validate these findings and assess the extract's safety and efficacy in clinical settings.
In conclusion, this study highlights the potential of JLE as a natural therapeutic agent for inflammatory skin diseases, demonstrating its ability to modulate key markers of inflammation and enhance skin barrier function in vitro. These findings provide a solid scientific basis for improving skin health and advancing the treatment of chronic skin disorders. However, further research is essential to validate these results. Future directions include in vivo studies to investigate the extract’s effects in a more physiologically relevant context and clinical trials to evaluate its safety and efficacy in human subjects. Such studies will offer critical insights into the therapeutic applications of JLE and its potential role in managing inflammatory skin conditions.
Acknowledgments
This work was supported by the Derived candidate of chronic respiratory disease by expanding the indication for Korean herbal medicines (KSN1823221) and Development of Sustainable Application for Standard Herbal Resources (KSN1823320) from the Korea Institute of Oriental Medicine.
REFERENCES
-
Boguniewicz, M., Leung, D. Y. M., 2011, Atopic dermatitis: A Disease of altered skin barrier and immune dysregulation, Immunol. Rev., 242, 233–246.
[https://doi.org/10.1111/j.1600-065X.2011.01027.x]

-
Elentner, A., Finke, D., Schmuth, M., Chappaz, S., Ebner, S., Malissen, B., Kissenpfennig, A., Romani, N., Dubrac, S., 2009, Langerhans cells are critical in the development of atopic dermatitis-like inflammation and symptoms in mice, J. Cell Mol. Med., 13, 2658–2672.
[https://doi.org/10.1111/j.1582-4934.2009.00797.x]

-
Gallegos-Alcalá, P., Jiménez, M., Cervantes-García, D., Córdova-Dávalos, L. E., Gonzalez-Curiel, I., Salinas, E., 2023, Glycomacropeptide protects against inflammation and oxidative stress, and promotes wound healing in an atopic dermatitis model of human keratinocytes, Foods, 12, 1932.
[https://doi.org/10.3390/foods12101932]

- Hijnen, D., De Bruin-Weller, M., Oosting, B., Knol, E. F., Bruijnzeel-Koomen, C. A. F. M., Clark, R. A., 2013, TSLP and IL-33 directly activate human skin-homing Th2 memory cells in atopic dermatitis, J. Allergy Clin. Immunol., 132, 1428–1430.
-
Hu, Y. Q., Zhang, J. Z., A., 2022, Comparison for type 2 cytokines and lesional inflammatory infiltrations in bullous pemphigoid and atopic dermatitis, Clin. Cosmet. Investig. Dermatol., 15, 2313–2321.
[https://doi.org/10.2147/CCID.S376845]

- Kim, M. S., Lee, J. W., 2021, Regulation of TARC and TSLP expression in keratinocytes: Implications for inflammatory skin diseases, Exp. Dermatol., 30, 382–392.
- Lee, J. H., Lee, D. U., 2018, Evaluation of anti-inflammatory properties of Juncus species in skin inflammation models, Phytomedicine, 45, 94–101.
-
Park, N. Y., Kim, S. G., Park, H. H., Jeong, K. T., Lee, Y. J., Lee, E., 2016, Anti-inflammatory effects of Juncus effusus extract (JEE) on LPS-stimulated RAW 264.7 cells and edema models, Pharmaceutical Biology, 54, 243–250.
[https://doi.org/10.3109/13880209.2015.1029053]

- Sugiyama, H., Shimada, S., Kitajima, M., 2010, Aquaporins in the skin: From physiology to functional approaches, J. Dermatol. Sci., 58, 1–8.
-
Uysal, P., Birtekocak, F., Karul, A. B., 2017, The relationship between serum TARC, TSLP and POSTN levels and childhood atopic dermatitis, Clin. Lab., 63, 1071–1077.
[https://doi.org/10.7754/Clin.Lab.2017.161107]

- Zeng, H., Lin, Q., Fan, H., Wang, Q., Gu, J., 2020, Role of aquaporin-3 in the pathogenesis of skin diseases, Int. J. Mol. Sci., 21, 9278.
Herbal Medicine Resources Research Center, Korea Institute of Oriental Medicine qp1015@kiom.re.kr
Herbal Medicine Resources Research Center, Korea Institute of Oriental Medicine gpark@kiom.re.kr
KM Convergence Research Division, Korea Institute of Oriental Medicine ssamggun85@kiom.re.kr
KM Convergence Research Division, Korea Institute of Oriental Medicine cozy11@kiom.re.kr